February 2023
MIT Chemical Engineers Develop Molecular Models Revealing the Breakdown of the Well-Known Nernst-Einstein Relation for Confined Electrolytes
What do ions like sodium and chloride found in simple table salt that we add to our food or that we encounter in biological ion channels have in common? They all move in a concerted manner in the presence of a suitable driving force. Indeed, when salt ions encounter a concentration gradient, they move from the region of higher concentration to that of lower concentration in a process called diffusion. Similarly, because salt ions are charged, they can be driven by external electric fields in a process known as electrophoretic motion or electromigration. For over 100 years, a ground breaking theory, known as the Nernst-Einstein relation, based on the seminal work of Walther Nernst and Albert Einstein, has advocated the notion that the processes of diffusion and electromigration are connected, where the two coefficients of transport, namely the diffusion coefficient (for diffusion) and the electrophoretic mobility (for electromigration), are linearly related. The Nernst-Einstein (NE) relation has generally stood the test of time and performs extremely well for a variety of aqueous salt solutions. However, in a recent study, MIT researchers along with collaborators at the Lawrence Livermore National Laboratory (LLNL), demonstrated that under certain conditions, the NE relation can breakdown by close to three orders of magnitude.
Movie highlighting a new mechanism of electrophoretic ion transport under confinement. Upon application of an external electric field along the CNT axis (i.e., along the z direction), the single file water chain is found to disintegrate, resulting in distinct K+ ion-water clusters, which then traverse the CNT at significantly higher velocities. Color code: carbon atoms in the CNT: blue, K+ : orange, Cl-: cyan, hydrogen in water: white, oxygen in water: red. Further, for clarity, the water molecules inside the CNT are shown differently than water molecules elsewhere and the lipid bilayer encapsulating the CNT is not shown.
These new findings were reported in the journal Nature Nanotechnology, in a paper by Dr. Rahul Prasanna Misra, PhD ’21 and Professor Daniel Blankschtein, the Herman P. Meissner (1929) Professor of Chemical Engineering, MIT, who carried out a theoretical investigation of ion transport through carbon nanotubes (CNTs) – one-dimensional cylindrical allotropes of carbon, in collaboration with experimental researchers at LLNL. “The diameter of the CNTs that we investigated is around 8 Angstrom, which allows only a single file of water molecules and ions to pass through them. To put this in perspective, the CNTs are about 50,000 times narrower than the average human hair strand. This enables unprecedented access and characterization of the molecular nature of water and ions under confinement,” explains Misra, who is currently a postdoctoral associate in the group of Professor Blankschtein. The MIT team of Misra and Blankschtein collaborated with LLNL researchers led by Dr. Aleksandr Noy, who pioneered an experimental setup known as the CNT porin (CNTP) system, and co-directed the project along with Professor Blankschtein. Further, the experimental measurements of ion transport were carried out by Dr. Zhongwu Li, a visiting researcher at LLNL, and the co-first author of the paper along with Dr. Misra. Other authors who contributed in performing the experimental measurements include: Dr. Yuhao Li, Dr. Yun-Chiao Yao, Ms. Sidi Zhao, and Dr. Yuliang Zhang from LLNL, and Professor Yunfei Chen from Southeast University, China. The CNTP system consists of 8 Angstrom diameter CNTs embedded in a lipid bilayer across which the transport of water molecules and salt ions can be precisely measured. Together, the MIT and LLNL teams carried out a combined theoretical and experimental investigation which shed light on how water molecules and salt ions move in a single file manner.
A long-standing puzzle in this area has been how aqueous salt solutions, or electrolytes, behave in such confined spaces. In the bulk solution, salt ions tightly bind water molecules mainly through electrostatic interactions, resulting in what is known as the hydration shell. However, when a salt ion approaches a solid from the bulk solution, additional interactions may arise. The starting point for Misra and Blankschtein’s current investigation goes back to Dr. Misra’s PhD thesis, supervised by Professor Blankschtein, where they developed a multiscale framework from first principles to describe how electrons in the solid interact with those in the salt ions and water molecules. For a long time, researchers assumed that the interactions of salt ions and water molecules with solids can be described using pair-wise additive interactions. This assumes that the ion/solid interaction can be isolated from the water/solid interaction. However, the electrons in the solid can fundamentally break this assumption, resulting in fully coupled ion/solid and water/solid interactions. As Blankschtein explains, “Because salt ions are charged and water molecules have a permanent dipole moment, both can exert finite electric fields which can polarize the charge distribution in the solid. The induced multipoles of the solid generated in response to these electric fields can then interact electrostatically with the salt ions and water molecules. In essence, the solid can be thought of as a mirror which reflects the electric fields exerted by the salt ions and water molecules back onto them. This creates a many-body effect and a type of intercommunication between the ions and the water molecules, whereby a salt ion upon approaching a solid can change the water/solid interactions, and similarly, water molecules can also significantly alter the ion/solid interactions.”
Misra and Blankschtein first applied their theory to investigate salt ion adsorption at planar solid/water interfaces, including the graphene/water interface which has shown a lot of promise for electrochemistry and membrane-based applications. By carrying out molecular dynamics simulations, they showed that salt ions interact very strongly with solids in vacuum (referred to as the intrinsic ion/solid interactions) due to the large ion/solid polarization energy resulting from the ion electric field-induced electronic polarization of the solid. On the other hand, the interfacial water molecules at solid/water interfaces were found to significantly attenuate the ion/solid interactions due to a vectorial cancellation of the electric fields exerted by the salt ions by those exerted by the water molecules. An accurate modeling of electronic polarization effects was found to be essential to describe the two scenarios, including explaining the mechanism by which salt ions get adsorbed to, or repelled from, solid/water interfaces. “After investigating planar solid/water interfaces, a natural follow-up challenge was to consider what happens when there are not enough water molecules to screen the ion/solid interactions,” says Misra. Such a scenario is indeed encountered when salt ions are confined in a single file arrangement inside very narrow CNTs.
When Misra and Blankschtein began modeling the behavior of water and ions in very narrow CNTs, the MIT and LLNL teams became part of a multi-PI collaboration – the Center for Enhanced Nanofluidic Transport (CENT), which is an Energy Frontier Research Center (EFRC) funded by the Department of Energy, Office of Science, Basic Energy Sciences. Members of CENT led by Professor Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT, published a perspective article identifying seven critical knowledge gaps in our understanding of mass transport, one of them being the nanoscale solvation behavior of ions under confinement. Therefore, CENT provided the ideal platform for the MIT and LLNL teams to work together on this challenging problem. “As part of this collaboration, our goal was to investigate nanoscopic solvation and ion transport using the theoretical tools which we developed for planar solid/water interfaces. This would allow us to test the ability of our theory to describe both the planar (unconfined) and the curved (confined) environments self-consistently,” adds Blankschtein.
Before a salt ion can squeeze into the interior of a CNT, it has to lose water molecules from its hydration shell, where the thermodynamic barrier for this process is referred to as the dehydration penalty. It has generally been thought that dehydration is the primary mechanism of ion transport and selectivity through nanopores, with some previous studies hypothesizing that smaller-diameter CNTs can fully block all salt ions from passing through them. However, had this been the case, then it would have been impossible to study any kind of ion transport through such CNTs. As Misra explains, “While the loss of hydration shell water molecules imposes a very stiff dehydration penalty, there is a counteracting stabilization of the salt ion resulting from the electronic polarization of the CNT. This is because in a single file arrangement, there are almost no water molecules in the radial direction to screen the electric field exerted by the salt ion. Accordingly, the significantly large ion/CNT interaction due to the polarization interactions is able to fully compensate for the dehydration penalty.”
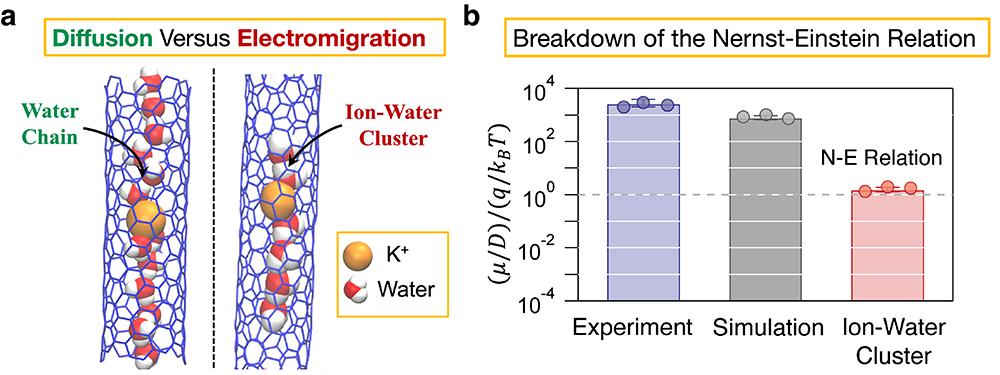
Using their theory, Misra and Blankschtein collaborated with the LLNL team to investigate ion transport through CNTs, showing that CNTs indeed allow cations such as the potassium (K+) ion to pass through them. The LLNL team used the fluorescence from a K+ ion-sensitive dye to measure the K+ ion permeability through 8 Angstrom diameter CNTPs, and carried out separate experiments using external voltages to obtain the K+ ion electrophoretic mobility. This was the first time that separate measurements of the K+ ion diffusion coefficient and electrophoretic mobility were carried out inside smaller-diameter CNTs. Further, the experiments were able to quantify the Nernst-Einstein (NE) ratio, defined as (μ/D)/(q ⁄ (kBT)), where µ is the electrophoretic mobility, D is the diffusion coefficient, q is the charge of the K+ ion, and kBT is the thermal energy. Intriguingly, the NE relation was found to break down by more than three orders of magnitude, with the NE ratio instead of being close to 1 (when the NE relation is satisfied) was found to be larger than 1000 inside CNTPs.
To uncover the molecular basis by which salt ions translocate during diffusion versus electromigration, Misra and Blankschtein carried out computer simulations which closely replicated the experimental conditions. It was found that when a concentration gradient is applied across the CNTP, the K+ ions diffuse extremely slowly, with the simulated mean squared displacement (a metric used to characterize diffusion) indicative of subdiffusion. Physically, the extreme confined space of the CNTP traps K+ ions such that the single file water chain does not provide enough room to the K+ ions to hop from one site to another during diffusion (see the schematic on the left in panel (a) of the figure below). Consequently, the effective diffusion coefficient of the K+ ions was found to be about 3 orders of magnitude lower than that in the bulk solution. In stark contrast, when simulations were carried out in the presence of external electric fields, the single file water chain was found to disintegrate, forming distinct ion-water clusters (see the schematic on the right in panel (a)), which then passed through the CNTP at significantly larger velocities. In essence, the K+ ions feel a disproportionately larger force due to the electric field compared to the charge-neutral and dipolar water molecules, which in turn aids in the disintegration of the single file water chain. Therefore, simulations predict that it is the dramatically different structuring of water around the K+ ions during diffusion versus electromigration which results in the large breakdown of the NE relation, consistent with the experiments (see the blue and grey bars in panel (b)).
Although the original derivation of the NE relation did not account for effects such as ion-ion correlations or nanoconfinement, these effects if at all present, have been traditionally thought to cause only a minor breakdown of the NE relation. To understand the breakdown in more detail, additional simulations were carried out to calculate the diffusion coefficient of individual ion-water clusters which were found to closely satisfy the NE relation (red bar in panel (b)). This corresponds to a hypothetical situation which cannot be replicated in experiments because ion-water clusters will not form during regular diffusion. Nevertheless, these simulations demonstrate that the NE relation is closely satisfied when both the diffusion coefficient and electrophoretic mobility are calculated for the same chemical environment. As Misra explains, “Our work shows that although the mathematical foundation of the NE relation remains valid, the overall relation breaks down because confinement induces fundamentally different mechanisms for diffusion and electromigration. This, in turn, points to the need for separately estimating the diffusion coefficient and electrophoretic mobility in strongly confined systems.”
Apart from being of fundamental importance, knowing when the NE relation breaks down can have many practical applications. For example, the confined interior of CNTPs resembles that of biological ion channels – transmembrane proteins, which one of its many functions is to transport ions across our cell membranes, and as such are critically important for the functioning of our neurons and cardiac cells. Some of these channels are voltage-gated, including the sodium and potassium ion channels, indicating that these channels allow more ions to pass through them upon encountering a voltage drop across the membrane. A better understanding of ion transport, including formulating more advanced theoretical models, could potentially accelerate the discovery of drugs for diseases involving ion channels. “Our research shows that confined environments provide the perfect scenario for electronic polarization effects to be dominant, and although our theory in its current form has been applied to CNTs, we believe that these effects can also be critical in other material architectures, including both synthetic (e.g., metal organic frameworks, boron nitride nanotubes) and biological channels,” says Blankschtein. Keeping in mind different applications of their work in electrochemistry, membrane science, and biology, the MIT and LLNL teams, under the umbrella of CENT, are continuing with their collaboration to investigate nanoscale ion transport involving different types of salt ions and nanopores.